Discover the essential filter integrity testing devices for maintaining the suitability and integrity of membrane filters in the industry.
Ensure product safety and regulatory compliance with Pall’s advanced filter integrity testing solutions. Designed for the food and beverage industry, our systems verify that integrity test filters are performing to specification—before and after use—without compromising filter structure or performance.
Why Filter Integrity Testing Matters
Filter integrity testing is a non-destructive method used to confirm that membrane filters are intact and capable of delivering the required microbial retention. It plays a critical role in:
- Validating sterilizing-grade filters
- Ensuring consistent product quality
- Supporting regulatory and HACCP compliance
- Reducing contamination risks and costly downtime
Trusted Integrity Test Filters and Devices
Pall offers a range of integrity test filters and automated test devices, including the Palltronic® Compact Touch, designed for ease of use, accuracy, and compliance. These systems support:
- Forward flow and pressure decay tests
- Reliable detection of filter breaches
- Fast, repeatable results for batch release and process validation
Benefits of Pall’s Filter Integrity Testing Solutions
- 100% of filters tested during manufacturing
- Compatible with sterilizing-grade and microbial reduction filters
- Supports both pre- and post-use testing
- Enhances traceability and documentation
The Pall Compact Touch (Integrity Test Device)
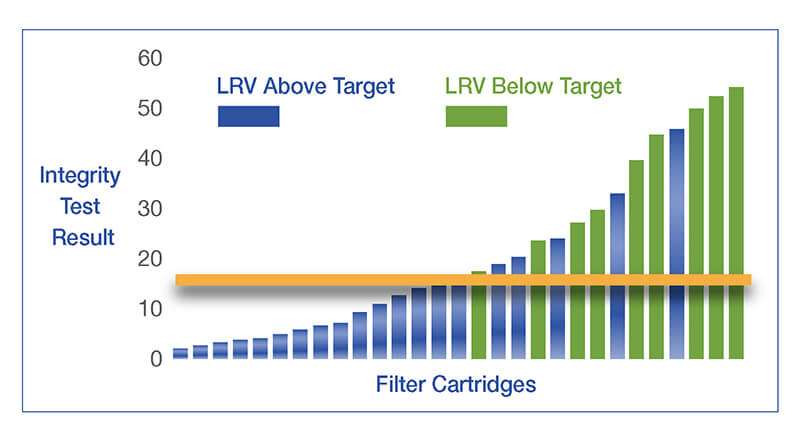
Recommended Integrity Tests to confirm the integrity of microbial reduction and sterilizing grade membrane filters
Two integrity tests are recommended to confirm the integrity of microbial reduction and sterilizing grade membrane filters: Forward and Pressure Decay. The value of forward flow is linked to the results of the bacterial challenge test. The pressure decay value is derived from the forward flow value and the volume upstream of the housing.
These tests correlate to a set microbial removal or reduction level during the initial filter validation. As non-destructive tests, they may be used after sanitization and at the end of a production batch to show the filter assembly is integral. These tests are based on the same principles governing the gas flow through a wetted pore.
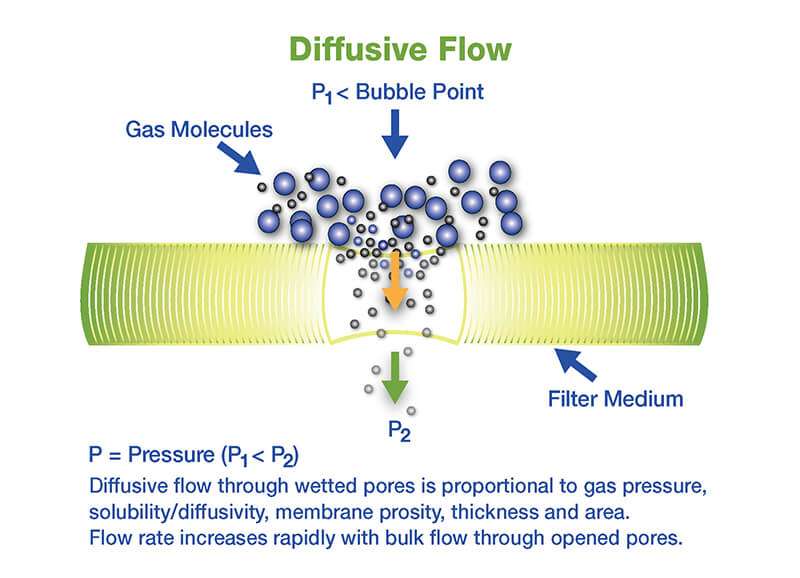
Forward flow test
- The pressure within the upstream volume of the filter housing is maintained at the filter-specific forward flow test pressure.
- The total gas flow, made up of diffusive flow through wet pores and bulk flow through open pores or defects, through the filter is measured by the test device upstream or downstream forward flow, which measures the flow required to maintain the test pressure.
Pressure decay test (also known as pressure hold)
- The pressure within the upstream volume of the filter housing is stabilized at the filter and housing-specific pressure decay test pressure.
- The test device upstream measures the total gas flow through the filter.
- Pressure decay measures the drop in the pressure.
Water for Intrusion Test (WIT)
- Water flow is measured through a hydrophobic filter and correlated to microbial retention.
Bacterial retention testing is the most sensitive integrity test for sterilization ability and microbial reduction. Testing for filter integrity can be destructive and may contaminate the filter with test bacteria, making it unsuitable for use. If properly correlated, non-destructive tests can indicate how well the filter retains bacterial challenges.
Practical Considerations When Performing Integrity Tests
| Selection of Integrity Test Fluid | Standard "Reference" Test Fluids
Water: Hydrophilic & Hydrophobic Filters
Alcohol/Water: Hydrophobic Filters
Process Fluids (derived values)
|
| Selection of Gas for Pressurization | Acceptable Test Gases:
Unacceptable Test Gases:
|
| Temperature | The temperature should stay within 5 °C during an integrity test. Significant temperature changes can alter the fluid's gas diffusion. The recommended temperature range for integrity testing is 20 °C ± 5 °C (68 °F ± 9 °F). A 1 °C temperature change will cause an approximate 0.3% volume change due to its critical impact on gas pressure. |
| Upstream Volume | The upstream volume is an essential consideration for Pressure Decay tests. In applications where filters are effectively regenerated, their service lives will be dictated by their structure integrity. |
Protect your process with proven filter integrity testing solutions. Explore Pall’s full range of integrity test filters and devices to ensure your filtration systems meet the highest standards of safety and performance. Contact our expert team today to see how we can help.
Palltronic® Compact Touch
Learn more about the next generation of pressure decay integrity test devices, designed specifically for Food & Beverage producers.