We can help you find the right cold brew filtration solution for your needs. Contact us for a free 30 minute filtration consultation.
Reducing Clostridium Botulinum Risk in Cold Brew Coffee Filtration
The global demand for cold-brew coffee continues to rise, with the market valued at $506.1 million in 2023 and projected to grow at a CAGR of 19.9% through 2030. Consumers are increasingly drawn to cold brew for its smooth, sweet flavor and refreshing ready-to-drink (RTD) appeal. However, as production scales, so does the need for stringent safety protocols—especially in cold brew coffee filtration.
Why Cold Brew Coffee Filtration Matters
Cold brew coffee is typically brewed using cool water over extended periods, which extracts fewer oils and acids. This results in a beverage with a pH above 4.6 and water activity greater than 0.852, classifying it as a low-acid food. These conditions can support the growth of harmful microorganisms like Clostridium botulinum if not properly managed.
This makes cold brew filtration a vital step in ensuring product safety and shelf stability.
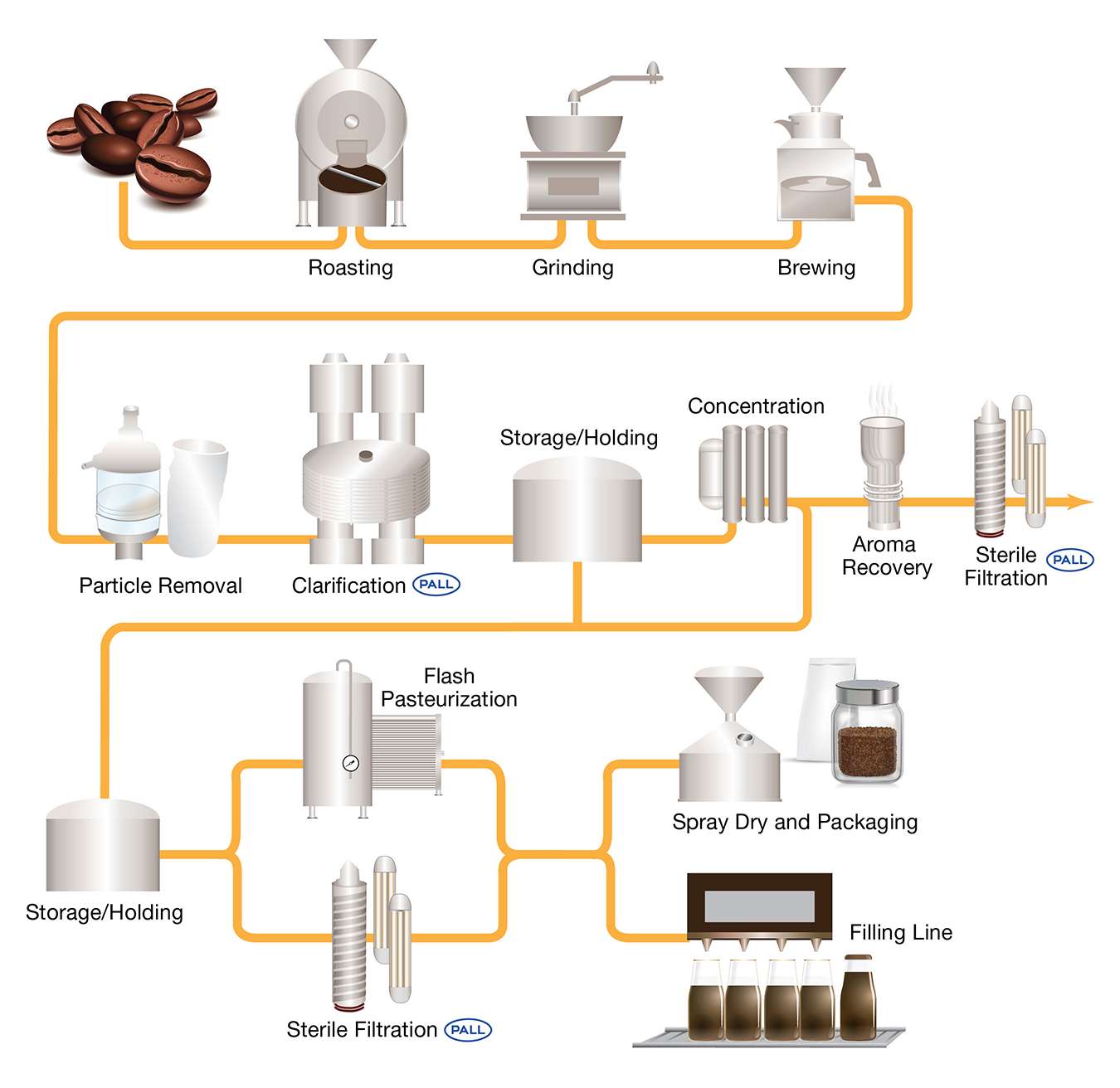
Process Diagram Of Cold Brew Coffee Filtration
Ensuring Safety Through Effective Cold Brew Filtration
To mitigate microbial risks, cold brew coffee filtration plays a critical role in both upstream and downstream processing:
Upstream Filtration: Removes coffee grounds and haze particles to clarify the extract.
Downstream Filtration: Targets microbial contaminants using advanced methods such as:
- Membrane filtration
- Pasteurization
- High-pressure processing (HPP)
These steps are essential to prevent the growth of C. botulinum and ensure product safety, especially for RTD cold brew products that may be stored unrefrigerated.
Meeting FDA Safety Standards
The Food and Drug Administration (FDA) mandates that all commercial processors of low-acid foods register their facilities and file validated processing methods. For cold brew coffee, this includes incorporating cold brew coffee filtration as a validated control measure in HACCP plans. This ensures that C. botulinum spores do not germinate and produce toxins during storage or distribution.
Best Practices for Cold Brew Coffee Filtration
To maintain safety and quality:
- Maintain cold chain logistics below 4°C (39°F)
- Incorporate multi-barrier filtration systems
- Monitor pH and water activity
- Implement a robust HACCP plan with validated microbial controls